Other questions on the subject. Double replacement reactions also result in the formation of a solid product which is called a precipitate.

Double Replacement Double Displacement Reaction
Combining vinegar and baking soda to create homemade volcano.
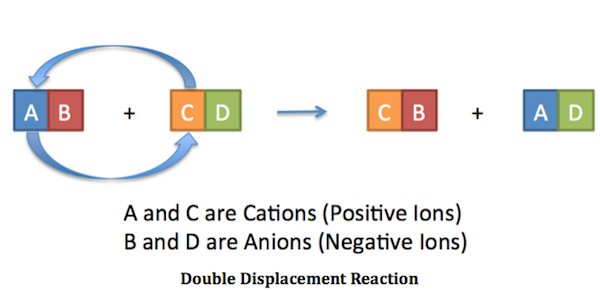
. A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative. What is a double replacement displacement reaction. The solvent for a double replacement reaction is usually water and the reactants and products are usually ionic compoundsbut they can also be acids or bases.
Chemistry 21062019 1700 paovue48. To quote from an online source. Stay tuned with BYJUS to learn more about other concepts such as displacement reactions.
A double replacement reaction will occur if a formation of a precipitate gas or water takes place. The following is a real life example of double replacement reactions. A Double displacementor double replacement rxn or metathesis occurs bcz when two ionic compounds react and the positive ions cation and negative ions anion of the two reactants must be ionic compounds exchange their.
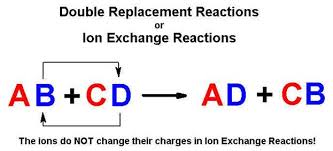
This is simply based on the solubility chart of inorganic compounds. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. A double replacement reaction is a chemical reaction where two reactant ionic compounds exchange ions to form two new product compounds with the same ionsDouble replacement reactions take the.
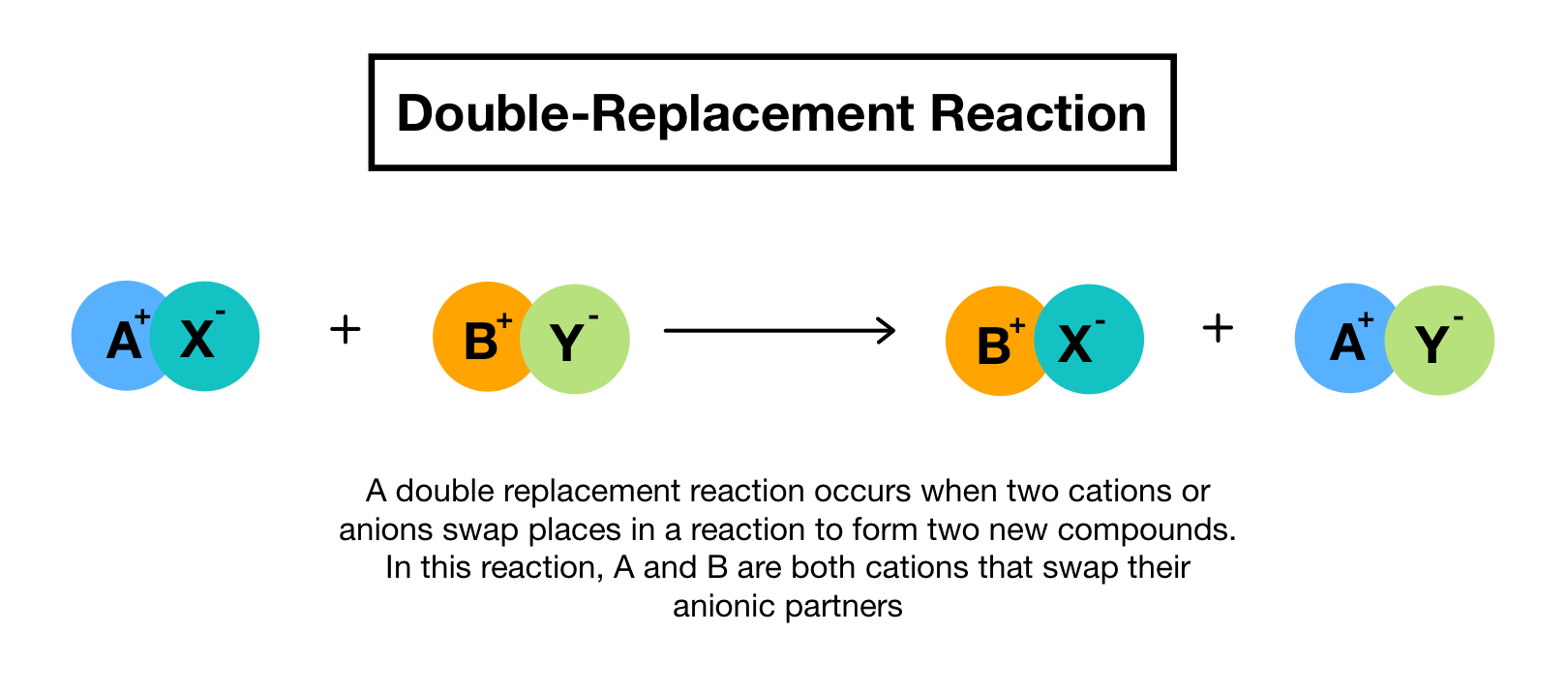
A type of reaction in which the ions of two compounds exchange places in an aqueous solution to form two new compounds Describe the nature of the reactants and products in a single replacement reaction. Double replacement reaction occurs when the cations and anions of two ionic compounds are exchanged. The reaction occurs most often between ionic compounds although technically the bonds formed between the chemical species may be either ionic or covalent in nature.
You can think of the reaction as swapping the cations or the anions but not swapping both since you would end up with the same substances you started with. A double-replacement reaction exchanges the cations or the anions of two ionic compounds. Molecular complete ionic and net ionic equations.
In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable. What is a double replacement. CuCl 2 aq 2 AgNO 3 aq CuNO 3 2 aq 2 AgCls.
When does a double replacement reaction occur. A double-replacement reaction occurs when parts of two ionic compounds are exchanged making two new compounds. A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products.
Double Replacement Reactions Fall 2017 page 4 of 9 Note that the physical state aqueousaq must be included to distinguish the acid from other forms of a substance. What is the formula for calcium iodide. Solubility rules are used to predict whether some double-replacement reactions will occur.
The following is the initial neutralization reaction. Chemistry 21062019 1700 paovue48. The figure below clearly illustrates how this swap takes place.
Molecular complete ionic and net ionic equations. Select two compounds above and this calculator will predict whether or not the reaction will occur in water. Double replacement sometimes referred to as double displacement reactions are when parts of ionic compounds are switched to form two new ionic compounds.
Two compounds react to form two new compounds. In double replacement reactions the elements get replaced in both the reacting compounds. What is the formula for calcium iodide.
2015 AP Chemistry free response 3a. The way I think of it since were dealing with ionic compounds is that when I write out a reaction I. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products.
This is the currently selected item. A double replacement equation has two reactants each composed of two species and they exchange partners to form either a. A double displacement reaction is also called a double replacement reaction salt metathesis reaction or double decomposition.
2 Get Other questions on the subject. The primary difference between a single and double replacement reaction is that in a single replacement reaction a free element is exchanged for another to yield a new compound and a new element. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
Molecular complete ionic and net ionic equations. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. For example the formula HCl can be used for hydrogen chloride gas HClg so to indicate hydrochloric acid one must specify HClaq.
In a double replacement reaction the components of two compounds are exchanged or displaced to form two new compounds.

How Do You Balance Double Replacement Reactions Socratic

What Is Double Replacement Reaction Example Share Education
Double Replacement Reactions Definition Examples Expii

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
What Is A Double Replacement Reaction In Chemistry Socratic


0 komentar
Posting Komentar